If you’re looking to lose weight, you may be hearing left and right that you need to count calories. Popular wisdom goes “calories in, calories out”, and a successful weight loss, we think, results from nothing more than satisfying a simple calculation: consistently consume fewer calories than you use up, say in a day.
Following this saying, it stands to reason that to lose weight we need to count calories. But then you’d think that something like a calorie has a straightforward meaning. It turns out that neither calories, nor consuming and using up those calories from food follow simple rules.
The history of the concept of calories hides a few clues to the origin of the nutrition science, and the health advice we hear everyday. Discovering these clues may help us to understand the really complex ways in which seemingly simple things like calories, macro nutrients, and diet affect our health and well-being.
We hope that in this article you’ll find out more about the “why” of certain food choices and feel empowered to build or maintain the habits you desire.
- How much energy does a Calorie contain?
- Calories come from France
- Nutrition science is born in the US
- Balanced diets for doctors and “workingmen”
- Which foods are good foods?
- Calorie counting: for health or money?
- Calories and the Atwater factors
- Calorimetry – why we “burn” calories
- Metabolisable energy and calories – two different things
- Macros are elusive to track
- Would you eat only flour for a week?
- Digestibility influences available calories
- The Atwater factors and food labeling
- 3 things calories won’t tell you
- TL;DR: Calorie counting is hard
- 3 simple guidelines to support clean eating
How much energy does a Calorie contain?
Quirky historical circumstances converged so that a Calorie (capitalised) came to indicate a a kcal (kilocalorie). A Calorie stands for a pretty big amount of energy, a kcal being a 1000 times the energy of an (uncapitalised) calorie. Imagine the energy needed to heat up a kilogram of water with 1 degree Celsius (or a pound of water with 4 degrees Fahrenheit) – that’s a Calorie.
The point is a Calorie is a huge amount of energy, and as we’ll see below, if you eat mostly raw fruits, vegetables, and grains – as our deep-past predecessors probably did – you’d have a hard time getting lots of Calories from foods.
On the flip side, if your fitness app assures you you’ve burned 150 Calories in 7 minutes, you should be raising an eyebrow – most workouts (and physical activity) will burn only 30-80 Calories for every 10 min (200-500 Calories/hour). Only swimming like crazy will probably get you in the 130-150 Calories/10 min range (800 Calories/hour).
Amazingly, these Calories, which have been used on nutrition labels in the US since, originally found their way to the US somewhat accidentally at the end of the 19th century. The person responsible for gifting the Calorie is one Wilbur O. Atwater, an American chemist, who brought the definition back to US from his post-doc studies in Germany.
Atwater also laid down the foundation of nutritional science in the US; before his time calories lived pretty much in the domain of physicists and had little to do with human food, diet, or physiology.
Calories come from France
Although consensus lacks still on who coined the word calorie, historical texts broadly agree that the term calorie originated in France, and from there made its way into other countries’ scientific communities – to the UK, Germany, the US etc. during the 19th century.
In “The History of the Calorie” James Halgrove notes that the word calorie was coined in the beginning of the 19th century, around 1819–1824, according to the 1995 Dictionnaire historique de la langue francaise (The Historical Dictionary of the French Language). Nicolas Clément, a successful French entrepreneur and industrial consultant, may have been the first to coin and define the calorie in 1819-1820.
Clément was not only a successful French entrepreneur and industrial consultant, but also held one of the first chairs in chemistry at the Conservatoire des Arts et Metiers in Paris. It was at the Conservatoire, in 1819, that Clément started teaching a course in industrial chemistry. In class notes taken by Clément’s students we find the first mentions of “calories”.

In any case, use of the calorie gained traction in the following decades, so by around 1845 the “calorie” as a unit of heat energy was being widely used by scientists and engineers. However, it would not be until much later, in 1887 that the Calorie – note the capital “C” – finally came into the common American nutritional vocabulary when Professor Wilbur O. Atwater introduced it in an article for Century magazine called The Potential Energy of Food. The Chemistry and Economy of Food.
The Calorie has, in fact, continued to define energy in human nutrition virtually since Atwater published in 1895 the Bulletin on Methods and Results of Investigations on the Chemistry and Economy of Food for the US Department of Agriculture.
Nutrition science is born in the US – end of 19th century
If Atwater did not pioneer calorie counting and macro-based nutrition in the US, he can certainly be said to have opened the door to both. After getting his doctorate in agricultural chemistry in 1869, Atwater spent years in Europe visiting “agricultural experiment stations”. He later learnt about “Calories” during his studies in Germany in 1882-1883.
The German school of physiology in those years was developing calorimetry to look into the most efficient sources of energy for animal feed, as well as nutritionally balanced human food. Atwater visited the laboratory of one German physiologist named Carl Voit who was using human and animal respiration to indirectly measure calories as early at 1866. Voit also experimented to find nutritionally balanced foods for both animal feed and human food.
Atwater spend in total 2 years in Berlin and Leipzig. He did postdoctoral research with another German physiologist, Max Rubner, who, in 1885 measured experimentally the caloric energy content of sugar, fats and egg white.

Atwater apparently was very much impressed by the approach taken by the German school towards nutrition. When he returned to the US from his studies, in an 1887 article in the popular Century magazine (part of a series), Atwater quotes a number of leading German physiological studies he had learnt about during his stay in Germany:
The respiration experiments have been made with dogs, in the Physiological Institute in Munich, by Dr. Rubner, who has also made an extended series with the calorimeter. The largest number of the experiments with food-materials in the calorimeter, however, have been conducted by Professor Stohmann, of the University of Leipsic, and his assistants. The results of experiments with the respiration-apparatus and with the calorimeter agree with most remarkable closeness. In supplying the body with fuel, the protein, fats, and carbohydrates, replaced each other in almost exact proportion to their heats of combustion.
In an excited footnote, Atwater acts as an enthusiastic ambassador of the latest scientific German knowledge to American readers:
Since these German researches are very recent and have not yet been made accessible to English readers, I could hardly expect to be excused if I did not give at least an inkling of the details.
Atwater then goes on to tabulate what even nowadays stands as the standard for the energy derived from food, and introduces the Atwater factors and the Calorie (in the sense of kcal) to the American public for the first time:
Taking out ordinary food materials as they come, and leaving out slight differences in digestibility, etc.[…] In one gram of protein, 4.1 Calories; in one gram of fats, 9.3 Calories; in one gram of carbohydrates, 4.1 Calories.
The Calories, which is the unit commonly employed in these calculations, is the amount of heat which would raise the temperature of a kilogram of water one degree centigrade (or a pound of water 4 degrees Fahrenheit).
In the same article of 1895 Atwater also lists the “Potential energy of food” as expressed by the “Calories in the nutrients in one pound of each food-material” for 44 common foods:
- from types of meat and fish defined in meticulous detail: beef sirloin, rather fat (1173 Calories); mutton, side, well fattened (1906 Calories), mackerel, average (696),
- to common products – hen’s eggs (760 Calories), rye flour (1614 Calories), oleomargarine (3679),
- and finally fruit and vegetables – pease (1476 Calories), sugar (1798 Calories), turnips (139 Calories).

It speaks to the complexity of the measuring food energy, even then, that Atwater attaches a number of qualifications to his list, including assumptions about the measurement of Calories, the water content of each “food-material”, as well as its “composition”:
These comparisons are for a pound of the whole edible substance of each material, including both water and nutrients.
But even if we were to compare equal weights of actually nutritive material, the differences would still be very wide [because of how much fat and carbohydrates they contain].
It is of course to be understood that the materials vary in composition and that these figures represent averages merely.
Balanced diets for doctors and “workingmen”
In 1894 Atwater wrote the first USDA Farmer’s Bulletin (No 23) entitled “Foods: Nutritive value and cost” where he illustrates the break down of some popular foods into “nutritive ingredients” – protein, fats, carbohydrates – which correspond to modern-day macro-nutrients. (macros)
In the same bulletin, Atwater defines “the nutritive ratio” – measured by “the ratio of the protein to the sum of all the other nutritive ingredients”. Using the nutritive ratio, Atwater classified foods as those with a “wide nutritive ratio” (containing little protein, and lots of fat and carbohydrates) or, conversely, food with “a narrow nutritive ratio” (those containing ample protein).
Examples of the wide ratio foods, Atwater said, were foods like “fat meat and potatoes”, whereas the narrow ratio group included foods such as “lean meat, codfish, and beans”. Sounds familiar?
Atwater didn’t stop at defining the nutritive ratio. He also advocated that either a very narrow or a very wide “nutritive ratio” could lead to an unbalanced, unhealthy diet – “either of these errors is disadvantageous.” And he evaluated the American diet already at the time as tending towards a wide nutritive ratio:
In other words, we consume on the whole relatively too little protein and too much of the carbohydrates and fats.
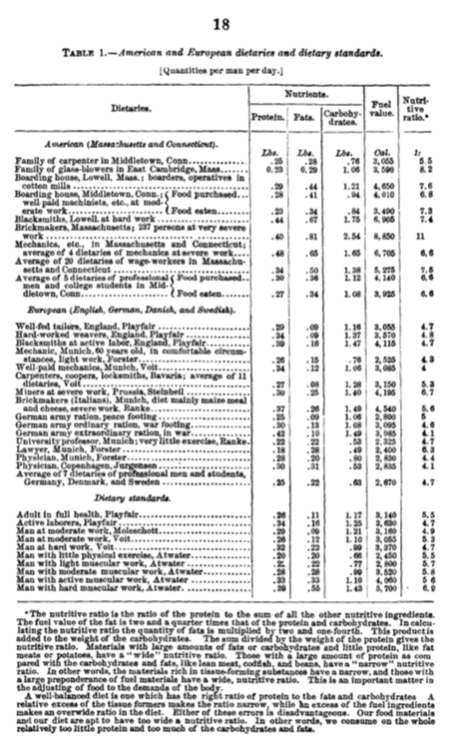
Which foods are good foods?
The definition of a balanced diet, however, remains more complex than a simple optimal number. When reporting on the “dietaries and dietary standards” to establish the food required by “different classes of people”, Atwater notes a wide range of energy requirements: whereas a physician in Munich or Copenhagen needed about 2800 Calories/day and a nutritive ratio of around 4, “brickmakers, in Massachusets, at very severe work” require a whopping 8850 Calories and a nutritive ratio of 11!
It’s important to understand the historical context of Atwater’s work. In the end of the 19th century, both in the US and Europe, it was customary even for people “in easy circumstances” to be spending minimum 50% of their income on food, with “workingmen” spending as much as 64% of the $350-400 they earned per year on food.
Atwater focused heavily on interpreting nutrition science findings within the context of what he called the “pecuniary economy of food”. He distinguished between cheap food (“ which supplies the most nutriment for the least money”), and economical food (which is the cheapest and at the same time best adapted to the wants of the eater).
The maxim that “the best is the cheapest” does not apply to food.
Atwater intended to educate ordinary people how they can best spend their money on the food they needed. His idea was to use food-nutrients per buck, so to speak, as a way to translate food prices for ordinary people to units they could understand and use in their food budgets.
Here’s the amount of smoked ham, butter, or corn meal you can get for 25 cents, and here are the actual amounts of “nutritive ingredients” – protein, fat, and carbohydrates – that you get for those 25-cents-worth.
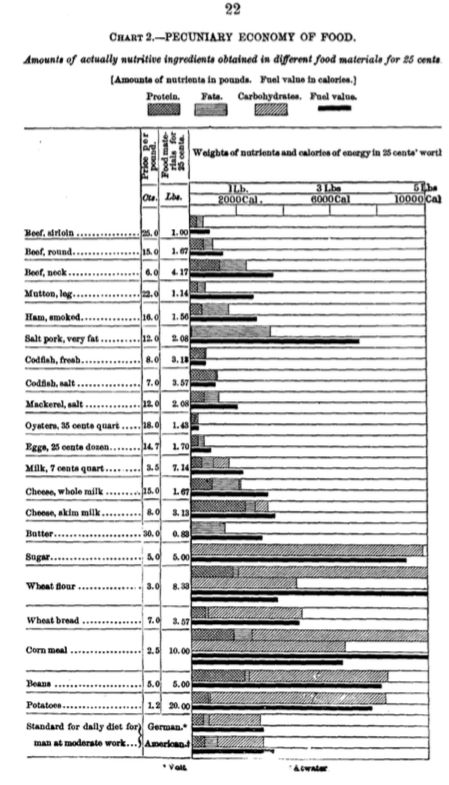
Calorie counting: For health or money?
Atwater went on to become the director of the Storrs Agricultural Experiment Station, and Special Agent of the United States Department of Agriculture, and in this role, he published a number of bulletins on food chemistry, food habits, diet and more, which pretty much set the stage for modern food science, calorie counting, and macro-nutrients regimens.
Dr. Lulu Hunt Peters introduced calorie counting to American in the best-selling ‘‘Diet and Health with Key to the Calories’’ and sourced a lot of information from Atwater. One reviewer from April 2013 on Amazon.com writes “And we thought counting calories was new“. Another reader in 2011 noted that “the book is very dated in some few respects, but shockingly modern in many others.”
Indeed, not only is calorie counting for weight loss not new, but the impact of money on health choices has remained strong since the end of the 19th century.

A 2005 study in the American Journal of Clinical Nutrition on the “economics of obesity” concludes that “Evidence is emerging that obesity in America is a largely economic issue.” The study classified food based on price, and “dietary energy density” (calories per gram), and found that healthy foods that contain fewer calories per gram (such as lean meat, fish, diary, fruits and vegetables) tend to cost the most.
On the other hand, the cheapest foods contain the most calories per gram, and tend to be less healthy (refined grains, added sugars, and added fats). The study suggests that higher rates of obesity among people with lower income have to do with the food they choose – cheaper, less healthy, and easier to consume on a tight time schedule.
Whether you agree or disagree that wallets determine diets, one important point is that upfront calories do not always determine what energy you’re getting from food.
Calories and the Atwater factors
Going back to the saying “calories in, calories out”, by now you may have noticed that things don’t quite stand so simple. Various factors do come between the eating of food and the using up of this food by the human body – via metabolism, exercise, or other physical or mental activities.
Understanding how scientists count calories, and how difficult that calorie counting has been, reveals the complexity of both food and the human body, and how the two interact. And the history also helps to realise that the whole calorie counting practice hinges on one important concept – the Atwater factors – the familiar correspondence of 4 Calories/gram protein or carbs, and 9 Calories/gram fat.
But first things first, let’s start with the basics. The 3 most important sources of energy for the human body from food are fats, carbohydrates, and protein. Whereas fats and carbohydrates contain carbon and hydrogen and get completely oxydised by the body into carbon dioxide and water, protein is special. Protein also contains nitrogen, and it is this link between nitrogen and protein that has allowed scientists to estimate the protein present in foods (we’ll get to this point again later).
Atwater developed a pretty sophisticated system to calculate the “available energy” from food. The legacy of this system are the Atwater factors, still used today in nutritional science and labelling. To convert macros to energy in food, estimate 4 Calories/g for carbohydrates and proteins, and 9 Calories/g for fats. To derive this simple calculation, though, Atwater conducted many studies in which he calculated calories using two different methods.
Calorimetry – why we “burn” calories
One method, called bomb calorimetry, employed a chamber-like device where a food sample is burnt completely. The heat resulting from the burn then gets transferred to water in the surrounding chamber, and the increase of the water’s temperature is used to calculate the calorie value of the burnt food sample – this is the heat of combustion, or in other words the gross energy of the food.
You might have guessed by now that bomb calorimetry gave us the expression to “burn calories”. Obviously, measuring food energy in this way differs a lot from the way the human body digests and uses food – be it to maintain its metabolism, or to build and repair body tissue such as muscles.

So Atwater developed another method to cross-verify the gross energy values derived from bomb calorimetry experiments. This other method for calculating calories in food attempts to take into account how food actually gets used up by the human body; it calculates what Atwater called available energy (nowadays known as metabolisable energy). Metabolisable energy is, of course, the calories we’d want to count if we are aiming to lose weight, maintain a certain weight, or even gain weight.
Metabolisable energy and calories – two different things
Figuring metabolisable energy depends on a seemingly simple arithmetic: take the total energy produced when the food sample burnt (the heat of combustion), and from this energy subtract the energy not used by the body. This unused energy, of course, we find in the loss of nutrients in “excreted matter” – urinary and fecal residues (urinary residues come into play only when we talk about protein, not fat and carbohydrates.)
However, some parts of excreted matter come from the normal metabolism of the body, so only a portion represents actually “unused” nutrients from eaten food. Distinguishing between the two allows us to understand how digestible the nutrients in various foods are – the more unused nutrients found in excreted matter, the less digestible the food is. Without knowing how digestible nutrients in foods are, we can’t count calories truthfully.
Macros are elusive to track
So Atwater and other scientists have been estimating this apparent digestibility of foods from excreted matter. Seems easy enough, but it turns out that macros – proteins, fat, and carbs, might be easier or more difficult to digest based on at least two factors: specific foods, and a person’s overall diet. Which is to say, yes, pretty much everything.
For example, when calculating calories for the protein contained in foods, we need to take into account a lot of additional factors:
- type of protein: not all protein is created equal and nutritional scientists widely accept that the nutritional value of proteins differs a lot depending on the amino acids they contain.
- whether foods are eaten raw, or cooked: even the method of cooking and the temperature can impact digestibility. Cooking foods like beans and legumes can make the protein and starch they contain 100% more digestible.
- other chemical components: for example, if there’s lots of insoluble fibre or “anti-nutritional factors” – like the tannins that legumes and cereal naturally contain – protein may also be more difficult to digest.
So, if two people are eating two foods containing the same amount of protein even assuming that their metabolisms are the same, they may well end up digesting different amounts of that protein. So the Atwater factors where fats equal automatically 9 Calories/gram, and protein and carbohydrates equal 4 Calories/gram does not reveal the full picture of the energy that our bodies can use from these foods – the metabolisable energy.
To improve the Atwater factors, in 1973, Annabell Merrill and Bernice Watt of the USDA experimented to modify and improve the Atwater system. They estimated more specific numbers (factors) to translate macros to metabolisable energy. The improvements took into account the various types of nutrients within each macro group of protein, fats, and carbs. They also put more focus on understanding how and why different macros can be more or less digestible depending on the food they come from.
Would you eat only flour for a week?
However, the studies of digestibility of foods are practically difficult and, by nature, very artificial. Basically, in such a study the diet of a person gets exclusively substituted with a single food for a period of time, usually at least a week. The researchers then measure how many calories of the specific food the person actually lost in their excretions. Multiple measurements taken from different people are then averaged to produce the final estimate of the digestibility for the specific food.
Let’s try to put ourselves in the shoes of the participants in one such study from the 1950s by McCance and Widdowson. The experiment sought to compare how digestible wheat protein was, so for that purpose they enlisted 6 volunteers who had to eat different types of wheat (English and Canadian Manitoba) flour only for a whole week.
[Diet] consisted largely of the bread under investigation. This was baked for us by Mr and Mrs Wilkin, Barrington, Cambs. Some of the flour was made into pastries and cakes. A little butter, some fat rendered out of bacon and small quantities of bramble and marmalade jelly were allowed. The only drinks permitted were water and weak tea without milk. Each person, if he remembered, took 50 mg. ascorbic acid daily. Thus for all practical purposes the flour constituted the sole source of N[itrogen] and furnished 77-930 of the total calories.
Ouch! Imagine being on a diet like this. Not unexpectedly, two out of the 6 volunteers experienced diarrhoea for 2 days during the study…

Also, as you can imagine, this approach assumes that foods behave the same way when eaten in isolation, as when they mix up with other foods in our intestine. Which leads us to the second factor that influences how much usable energy our bodies get from macros: overall diet.
From a technical point of view, simply how much protein a person takes overall can influence “apparent digestibility”. For example, if someone eats little protein (so, little nitrogen), the nitrogen found in their excretions must be coming mostly from metabolic processes, so there must be little unused protein.
Testing how digestible the protein in one specific food then becomes almost impossible: we’re trying to estimate protein from nitrogen, but nitrogen intake (from other protein-containing food) affects the excreted nitrogen!
In fact, Atwater experimented with both single foods and with the same foods included in a basic diet, and found that the apparent digestibility that he estimated from single foods needed to be revised because the foods were not behaving in the same way when they were combined with other foods in a diet.
Digestibility influences available calories
The improved Atwater factors proposed by Merill and Watt in 1973 establish a wide range of digestibility for protein of 20%-97%, for carbs of 32%-98%, and a narrower one for fats (90%-95%). In other words, lots of the macros – specifically protein and carbs – present in our food may end up unused by our bodies.
For example, depending on the specific foods you eat, and your overall diet, you may be using up only part of the calories in the protein you eat. Which is exactly what one study from 2005 found. Traditional diets from countries such as India, Guatemala, and Brazil contained proteins that are much more difficult to digest (54-78% digestible), compared to protein in typical North American diets (88–94% digestible).
Recently, some studies have proposed also that the range of digestibility for fats from various nuts like almonds, walnuts, pistachios etc. turn out to be lower than estimated by the Atwater factors, original or improved. For example, research by the USDA Agricultural Research Service and Beltsville Human Nutrition Research Center estimated that because of lower digestibility raw almonds contain 32% fewer metabolisable calories than labelling indicates (based on the Atwater factors). Similarly, for cashews (16% fewer calories), walnuts (21% fewer) and pistachios (5% fewer).

Atwater factors seem to overestimate not only calories from specific raw foods, but also for entire diets. One study from 2007 in the American Journal of Clinical Nutrition finds that the Atwater factors overestimate by 11% the energy available to the body from a high-fiber fruit and vegetable diet.
The Atwater factors and food labeling
Since Atwater laid the foundations of nutrition science at the end of the 19th century, a lot of progress has been made into understanding how the human body uses up food for its needs. However, you may be surprised to hear that, with some qualifications and modifications, the original Atwater system remains widely and largely in use today.
You can see Atwater’s vast legacy in nutrition labelling in the US and around the world. One place where we find traces of Atwater’s pioneering days are Food Composition Data (FCD) tables – detailed references to the nutrients and energy values of common raw and processed foods.
Some of these require paid access (Germany), and others are free to access publicly like the USDA National Nutrient Database for Standard Reference or the Danish Frida database (also available in English). Upon consulting them, you’ll discover that carbohydrates, for example, are listed in two ways: “carbohydrate by difference” and “carbohydrates available”.
The first measure was introduced way back by Atwater. You obtain “carbohydrates by difference” by deducting crude protein, fat, moisture, and ash from a 100 grams of the specific food. The latter measure is of course based on the “available energy” or calories from food – in other words, the metabolisable energy.
As an illustration, the Danish open-source food database Frida lists for 100 grams of raw carrots (Daucus carota L.) 9.0 grams of “carbohydrates by difference”, and 6.3 grams of “carbohydrates available”. The 3 gram difference in the two is due to dietary fibre being included in the first, but not in the second. And then there is the third measure: “carbohydrate for nutrition labelling” which is a different number: 6.1 grams.
This gap between the number for “-by difference” and “-for nutritional labelling” results from the so-called Protein Conversion Factor (PCF) – another legacy of Atwater’s system. PCF represents the number by which nitrogen should be multiplied to obtain the protein contained in food. Atwater suggested to use the predominant protein in a food to estimate the protein in the food, and specifically the number 6.25 because a lot of commonly occurring proteins contain about 16% nitrogen.
However, this strategy over-simplifies things again. Research as early as 1941 studies found that the nitrogen in different types of protein (say almonds vs. avocado) varies a lot, yet the Atwater factor of 6.25 continues to be widely in use in modern-day Food Composition Data tables by default, as “a fixed factor”. According to the Frida database documentation:
Available carbohydrate and carbohydrate for nutrition labelling may have different values. This results from using a fixed factor for converting nitrogen to protein of 6.25 for nutrition labelling purposes, while using different factors for different foods for scientific purposes.
To make a long story short(er), calculating macros in foods, and then converting those macros to calories requires a lot of intermediary steps, complicated assumptions and qualifications.
Also the methods of calculating seemingly straightforward numbers like the amount of dietary fibre in foods may vary or be evolving constantly (quoting from the Frida database documentation again):
There is no clear definition of dietary fibres. [our emphasis] The definition originally accepted is physiologically based and states that dietary fibres are plant polysaccharides and lignin, which are not degraded by human digestive enzymes. Danish dietary fibre values are based on the AOAC (Association of Official Analytical Chemists) method AOAC 15th Ed, 1990, 985.29. […] Old British dietary fibre values are traditionally based on the Southgate method, which usually gives somewhat higher values than the AOAC method, while newer values are based on the Englyst and AOAC
methods. […] Older American values are based on the ‘crude fibre’ method (1342 and 1346), which gives lower values than AOAC numbers, while newer American numbers are based on the AOAC method [Source 1938 and 2055].
More specific knowledge on macros and food energy is not always incorporated into food labelling (though you can often find it in Food Composition tables, where it’s listed for “scientific purposes”).
In fact, mostly the original “simple” Atwater factors (and sometimes the improved ones from 1973) determine the labelling of calories in food around the world. And the calories found on the nutrition labels are exactly the calories that the vast majority of people use to count calories.
3 things calories won’t tell you
Not only may the labelling (primarily of raw foods) overestimate the calories that the human body can actually digest and use, but also, the calories themselves do not indicate a bunch of other important factors.
1. Micronutrients: in addition to the 3 macros, micronutrients such as organic acids, minerals, vitamins etc. are needed for our health and proper body functions. Calories cannot give us any idea about those: for example, a quantity of calories from fat in butter or meat contains fewer micronutrients than fat from nuts.
A realistic non-laboratory month-long experiment found that French women who consumed either savoury biscuits or pistachios as an afternoon snack did not end up weighing differently, but those who ate pistachios also got their thiamin, vitamin B6, copper, and potassium.
2. Energy density: some studies suggest that how densely packed calories are may in itself influence how digestible calories are, as well as feelings of hunger and satiation. While calories per portion can give us an idea about the energy density of foods, counting calories in and of itself does not really help with choosing low density foods.
Independent of fat content, low energy dense diets generate greater satiety than high energy dense diets, suggesting that an important regulatory signal may be the weight or volume of food consumed.
3. Overall diet: calorie counting may be a great aid to get used to portion control, and in this sense, it can help with achieving desired weight and health goals. However, relying too much on calories can have a negative effect if it causes us to overlook our overall diet. Foods come from living organisms with incredible complexity, and over the centuries we have added to the complexity with various methods of preparation, which have allowed us to enjoy food in uncountable ways. The drawback that comes with this complexity stems from how difficult it is for controlled scientific research to grasp all the interactions of different foods and their effects on the human body.
A recent study of high-fibre diets in men found that all existing systems used to assess energy from food overestimated the available calories. The higher the % of fibre in the diet was, the higher the overestimation of digestible calories, but only for diets aimed at losing weight, not to maintain it.
Calories should support our mindset about health, not define it.
TL;DR: Calorie counting is hard
If you’ve read this far, you’re probably coming to the conclusion that calorie counting is a complex business, and precise numbers remain elusive.
Particularly food labelling often relies on simplifications even in basic things such as calculating the amount of protein in food.
It’s useful to know the calories in a food, but as much as calorie counting relies on knowing the calories quite accurately, any calorie counting is bound to be approximate (because of fuzziness in the calculations of the underlying macros, digestibility factors, and interactions of foods, to say nothing of individual genetics!).
So, what alternative or additional approach to calorie counting can you take?

3 simple guidelines to support clean eating
To make things clear, we’re not advocating against calorie counting really, you should definitely use whatever information you find useful to achieve your health goals.
The following rules of thumb may be helpful when estimating your calorie intake in a more intuitive way, perhaps as a supplement to formal calorie counting:
1. Raw vs. cooked: Raw foods tend to be less digestible than cooked ones, so if you want to lower your metabolisable calories, eat raw foods. Vice versa, cook your foods (for example, roast nuts) if you want to increase the metabolisable energy you get from the food. Note: for some tough foods like mushrooms cooking is always preferred to access nutrients.
2. Low-density vs. high-density foods: Low density foods, such as fruits, vegetables, and whole grains may signal fullness to the body, and lead to spontaneously eating less food.
3. Easy to digest vs. hard to digest foods: somewhat overlapping with the previous points, by eating foods whose macros are more difficult to digest completely you will likely “lose” more of those calories declared on the label. Generally, fruits, vegetables, legumes, and cereals tend to provide fewer readily digestible nutrients than meat, fish, eggs, and dairy.
Your body and foods form a complex system, so go easy on any changes in your diet. Think of your body and the energy it uses, along with the food you consume as a system that is not yet well understood. Why not approach your food choices with intuition, but also as a mini research into your specific body and needs? We wish you to stay curious, and enjoy the gift of nourishment!
Were you aware of how the Calorie was invented? Are you using any rules of thumb and/or calories counting in your eating habits? Please share your thoughts in the comments 🙂

